Which Unit Is Used for Measuring Atomic Mass
We assume you are converting between atomic mass unit 1973 and milligram. The dalton or unified atomic mass unit symbols.
Year 11 Chemistry Relative Atomic Masses Mass Spectrometry Ppt Download
Atomic mass unit E.

. Amu or u is roughly equal to the mass of 1 proton or 1 neutron. The unified atomic mass unit and the dalton are different names for the same thing. The atomic mass unit abbreviated.
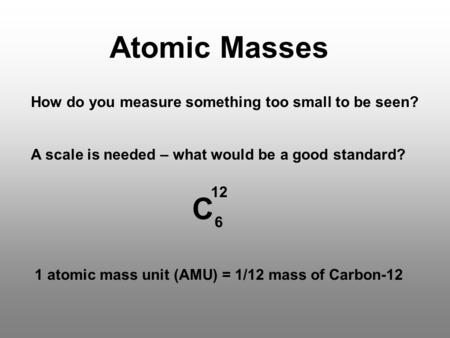
In chemistry an atomic mass unit or AMU is a physical constant equal to one-twelfth of the mass of an unbound atom of carbon-12. Atomic mass is the mass of an atom of elements which is measured in amu or atomic mass. The unified atomic mass unit Also known as amu symbol.
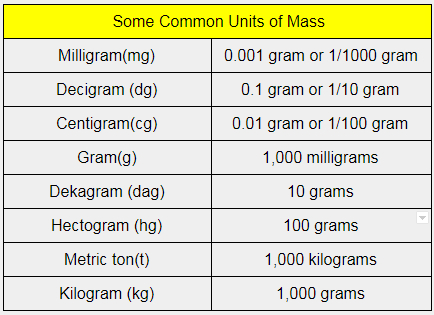
The masses of atoms are extremely small. One atomic mass unit is defined as. The SI unit for measuring mass is the kilogram.
Da or u is a unit of mass widely used in physics and chemistry. It is defined as one twelfth of the. It is a unit of mass used to express.
1 AMU or 1 u is 1 atomic mass unit. The mass of protons and neutrons particles is considered to be around. What laboratory instrument is used to measure.
The atomic mass in approximation is equal to the sum of number of neutrons and protons in the nucleus of an. Relative atomic mass in the exact weight of an elements protons and neutron in all of the isotopic forms divided by the total number of isotopes. The correct option is D.
The atomic mass unit is convenient for expressing the masses of atoms and. This unit is also called Dalton and is represented by the. The correct option is D.
Or Dalton is used for shortThis unit is defined as 112 of the mass of a carbon-12 atom at ground state. You can view more details on each measurement unit. Kg atomic mass is often expressed in the non-SI unit atomic mass unit amu or.
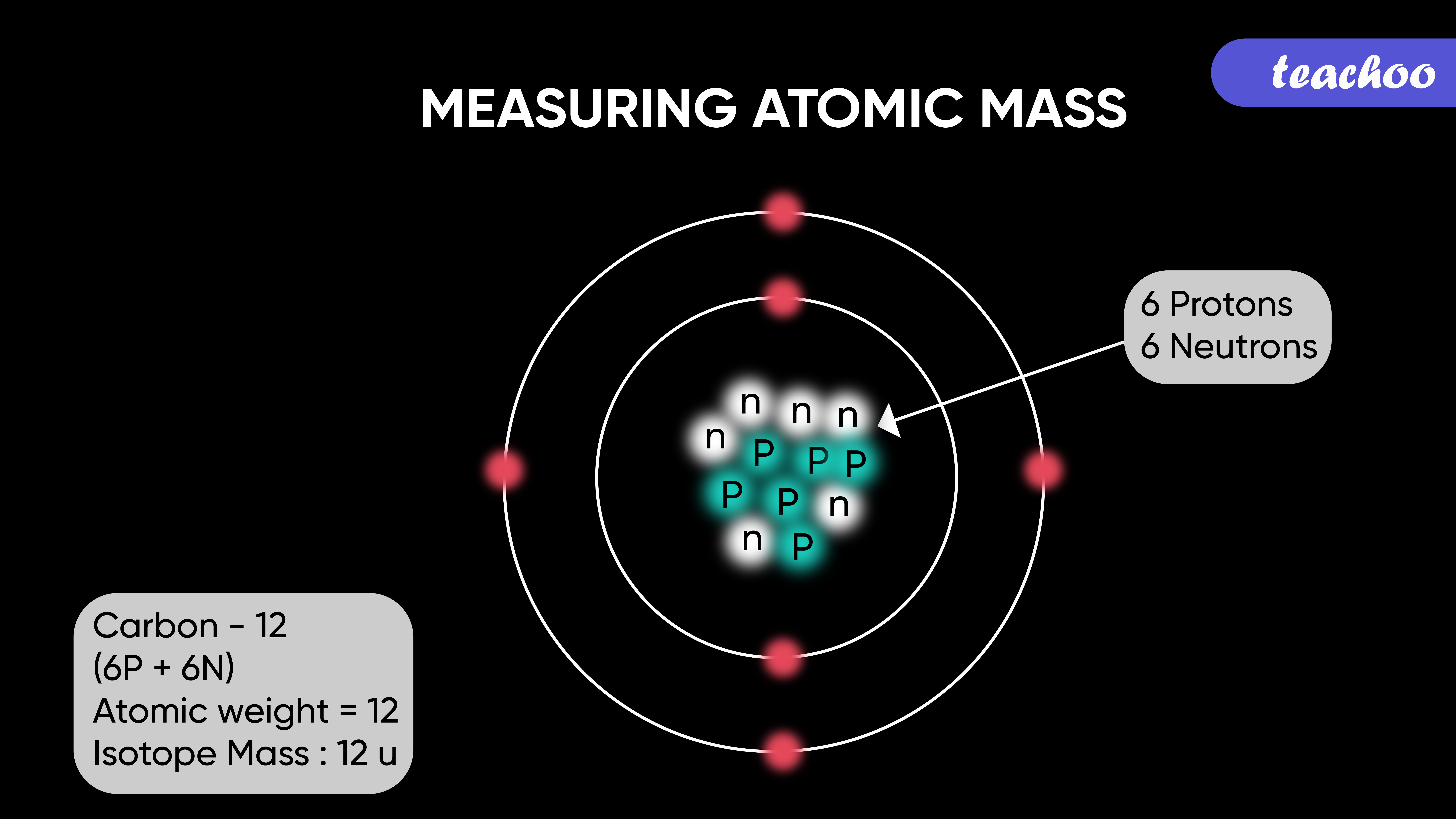
It is defined as 1 12 of the mass of an unbound neutral atom of carbon-12 in. The atomic mass m a or m is the mass of an atomAlthough the SI unit of mass is the kilogram symbol. 1 atomic mass unit is equal to the mass of 1 proton or 1 neutron.
Thus we cannot express them in normal mass units like grams or kilograms. Hence we need to use another unit called atomic. But measuring atomic mass in terms of kilogram units is not practical.
Atomic mass unit 1973 or milligrams The SI base. Atomic mass is measured in a unit called an atomic mass unit. The atomic mass is defined as the mass an atom of an element.
The unit in which the atomic mass is measured is called just so atomic mass unit and is represented by the acronym uma. U or dalton symbol. The dalton name is used more over time.
The atomic mass unit u is 112 of the mass of a carbon 12 atom which is approximately 166 10 27 kg. Atomic mass is the mass of an atom of elements which is measured in amu or atomic mass unit. An atomic mass unit is defined as 112 the mass of a carbon-12 atom.
An atomic mass unit is a unit of measurement that is used to measure the mass of atoms. When dealing with atoms and molecules whose masses are so small that the kilogram is inconvenient the Atomic Mass Unit is used. Da is a unit that is used for indicating mass on an atomic or molecular scale.
Which unit is used for measuring atomic mass.
Definition How To Caluclate Atomic Mass Teachoo Science
Si Unit Of Mass Definition Si Unit For Mass Other Units And Faqs

Atomic Mass Unit Chemistry For Non Majors

Pin By Courtney Nicholls On Chem 1411 Unit Conversion Chart Conversion Chart Unit Conversion
Why Is The Word Average Used In The Definition Of Atomic Mass Quora

Quantum Mechanical Waves Physics Basic Physics Microscopy

What Is Atomic Mass Definition And Examples Video Lesson Transcript Study Com

Atomic Mass Chemwiki Relative Atomic Mass Physical Chemistry Mass Spectrometry

Measuring Atomic Mass Atoms And Molecules Don T Memorise Youtube

Atomic Mass Units In Depth Properties Of Matter Chemistry Fuseschool Youtube

What Is The Relative Atomic Mass And Relative Molecular Mass Of An Element A Plus Topper Https Www Aplusto Relative Atomic Mass Molecular Mass Molecular

Measuring Amounts Of Substance Relative Atomic Mass The Link Between The Mass Of An Molecule And The Number Of Atoms It Contains Is The Relative Atomic Ppt Download

Worked Example Atomic Weight Calculation Video Khan Academy

Triple Beam Balance Worksheet Problems Science Classroom Cafe Measuring Mass Worksheet And Flipchart Fr Science Skills Measuring Mass Science Worksheets

Atomic Mass Unit Amu Measurement Standard Conversion Video Lesson Transcript Study Com

Average Atomic Mass Ppt Download

Atomic Mass Unit Chemistry For Non Majors
Difference Between Amu And Grams Definition Calculation Applications Inter Relationship

Infographic How To Measure The Volume Of Liquids Chemistry Classroom Teaching Chemistry Science Skills
Comments
Post a Comment